投资人关注的好项目上新了!BIOCHINA2026路演最新项目露出
2026年2月3日 10:00
BIOCHINA2026(第十一届)易贸生物产业展览将于3月12-14日在苏州盛大举行!现场将汇聚30000名生物产业同仁,数千名投资人、Biotech创始人、Pharma C-level及BD负责人齐聚一堂,高效对接项目、洽谈合作。
BIOCHINA2026期间,BiOFUNDING易企融资路演设置了AI制药、体内CAR-T、生物制造、科研、韩国项目路演等专场,深度聚焦生物医药领域前沿趋势与核心发展方向,为优质项目与资本精准对接搭建专业平台。
扫码注册门票
享多重福利
咨询路演详情
联系小易约见

继首轮优质项目官宣后,最新一批路演项目正式上线!有突破技术瓶颈的早期创新成果,也有具备临床转化潜力的项目,覆盖AI制药、体内CAR-T、核酸药物、细胞与基因治疗、小分子等赛道。
最新路演项目一览
项目名称/Project Name:
新一代AI驱动小分子设计平台及创新药开发
融资轮次/Funding Rounds:
天使轮
项目亮点/Project Highlights
我们致力于通过下一代人工智能重塑药物发现。由具有MNC背景的资深计算化学科学家领衔搭建的包括AI科学家,生物学家与药物化学家跨界组成的药物研发团队,开发国际领先的AI药物设计引擎,深度融合小分子生成式AI与物理计算,构建了分子生成与高通量自动合成优化相结合的端到端药物开发平台。目前已成功推进多个First-in-Class候选化合物进入临床候选阶段,显著缩短研发周期、降低开发成本、开启AI赋能新药研发的新纪元。
项目名称/Project Name:
人工智能大模型驱动的蛋白质设计
融资轮次/Funding Rounds:
A轮
项目亮点/Project Highlights
项目凭借自研蛋白质大模型,开创“AI设计+少量实验”新范式,解决研发周期长、多指标优化难等痛点。提供从设计、验证到商业化生产的全流程方案。已交付大量案例,覆盖医药及合成生物领域,获头部客户认可及多轮投资。
Project Highlights:
Project: AI-Driven Protein Design Leveraging a proprietary protein foundation model, this project pioneers an “AI Design + Minimal Validation” paradigm, solving bottlenecks in R&D cycles and multi-objective optimization. We provide end-to-end solutions from design to commercial production. With 30+ industrial cases delivered in biopharma and synthetic biology, our technology is validated by industry leaders and top-tier VCs.
项目名称/Project Name:
基于抗体非化学定点偶联LNP技术的in vivo CAR-T
融资轮次/Funding Rounds:
天使轮
项目亮点/Project Highlights
1.差异化tLNP靶向递送平台
•抗体和PEG设计&改造,实现高效偶联,效率高出传统马来酰亚胺—巯基偶联法数倍;
•平台化技术,针对不同靶点设计体内细胞药物;
•非化学的偶联,LNP结构稳定,工艺简单易放大,可实现in vivo CAR T量产
2.in vivo CAR T体内&体外高转染效率&杀伤能力
•细胞和动物体内均表现出T细胞高特异性转染;
•细胞和动物均保持高转染效率,转染效率/CAR T阳性率>80%,优于披露的同行水平;
•in vivo CAR T体外&体内超强的CD19+细胞(B细胞、NALM6细胞)清除能力
3. 适应症&管线:
•聚焦B细胞引起的自免;
•基于tLNP的in vivo CAR-T ,实现CAR T向标准化药物的转变; 具备“平台型创新药属性”,可开发不同 “体内细胞药物”
Project Highlights:
1.Differentiated tLNP Targeted Delivery Platform
•Engineered antibody and PEG design for highly efficient conjugation—several-fold higher than traditional maleimide-thiol coupling methods;
•Platform technology enabling design of in vivo cell therapeutics for diverse targets;
•Non-chemical conjugation ensures stable LNP structure; simple, scalable process enables mass production of in vivo CAR T.
2.High In Vivo & In Vitro Transfection Efficiency & Killing Capacity
•Highly specific T cell transfection demonstrated in both cellular and animal models;
•Maintains high transfection efficiency in both cells and animals, with transfection efficiency/CAR T positivity rate >80%, surpassing disclosed peer benchmarks;
•Superior in vitro and in vivo clearance capacity against CD19+ cells (B cells, NALM6 cells).
3.Indications & Pipeline:
•Focused on B cell-mediated autoimmune diseases;
•tLNP-based in vivo CAR-T transforms CAR T therapy into a standardized drug product; with "platform-type innovative drug" attributes, enabling development of various "in vivo cell therapeutics."
项目名称/Project Name:
mRNA的靶向递送技术及in vivo CAR-T的临床试验研究
融资轮次/Funding Rounds:
天使轮
项目亮点/Project Highlights
团队自研LNP载体In vivo CAR-T产品,核心研发指标突破显著、优于行业水平,构建坚实技术壁垒与清晰研发管线。项目亮点:
灵长类动物模型中CAR+T细胞生成效率与持久性领先;支持重复给药且安全可控,剂量优化灵活;多种模型中实现持久B细胞清除,疗效稳定;专有tLNP工艺保障规模化生产;CD19靶点产品已获IIT初步良好数据,助力后续临床推进与商业化落地。Project Highlights: The team has independently developed an LNP-vectored in vivo CAR-T product with notable breakthroughs in core R&D metrics that outperform industry benchmarks, forging a robust technological moat and a well-defined R&D pipeline.
Key achievements include:
- Leading efficiency and durability of CAR+T cell generation in non-human primate models;
- Enabling repeat administration with a safe, controllable profile and flexible dose optimization;
- Achieving sustained B-cell depletion with consistent efficacy across multiple preclinical models;
- A proprietary tLNP manufacturing process supporting large-scale production;
- The CD19-targeted candidate has yielded positive preliminary IIT data, laying a solid foundation for subsequent clinical advancement and commercialization.
项目名称/Project Name:
基于mRNA工程的体内Car-X破局之道
融资轮次/Funding Rounds:
Pre-B轮
项目亮点/Project Highlights
自2017年以来十多款CAR-T产品获批上市,不仅革新了血液肿瘤的治疗模式,还在实体瘤中显示出良好的应用前景。然而,传统的CAR-T细胞疗法依赖病毒载体在体外对自体T细胞进行嵌合抗原受体(CAR)的基因修饰,存在诱发次级肿瘤的风险。
本项目基于成熟的mRNA医药平台,针对性开展了靶向递送和序列优化工作,已实现对T、NK、Mφ等细胞的体内递送,完全避免基因组整合,提升治疗安全性,体内转化效率高达80%,支持新一代过继性细胞疗法的高质量开发与产业转化。
Since 2017, more than ten CAR-T products have been approved for market launch. This has not only revolutionized the treatment mode for hematological tumors, but also shown promising application prospects in solid tumors. However, the traditional CAR-T cell therapy relies on viral vectors to genetically modify autologous T cells in vitro, which poses the risk of inducing secondary tumors.
This project is based on the mature mRNA pharmaceutical platform and has specifically carried out targeted delivery and sequence optimization work. It has achieved in vivo delivery of T, NK, Mφ and other cells, completely avoiding genomic integration, improving treatment safety, and achieving an in vivo conversion efficiency of up to 80%, supporting the high-quality development and industrial transformation of the next generation of adoptive cell therapies.
项目名称/Project Name:
非动物源单细胞重组外泌体技术及其智造产业化平台
融资轮次/Funding Rounds:
A+轮
项目亮点/Project Highlights
依托成熟的合成生物学技术构建非动物源单细胞底盘种质资源库,通过基因工程改造与内源性装载设计,建立Pro-ExoX 重组外泌体平台及功能开发平台,实现非动物源单细胞外泌体递送、载药系统及规模化制备技术突破。结合代谢通路优化开发微藻/微生物基单细胞生产平台,实现高值化合物如虾青素、EPA、DHA等高效合成与生产。产品覆盖药物装载、健康消费双场景,技术壁垒深厚且产业化潜力显著,打造合成生物学在医药健康领域的规模化应用标杆。
The non-animal-derived single-cell chassis germplasm resource library has been constructed which utilizing mature synthetic biology technologies.Through the combination of genetic engineering and endogenous cargo loading design,the Pro-ExoX recombinant exosome platform and functional development platform have been established. This breakthrough enables non-animal-derived single-cell exosome delivery systems, drug-loading technologies, and scalable production methods.The single-cell production platforms based on microalgae/microorganisms which through integrating metabolic pathway optimization had been developed for the efficient synthesis and production of high-value compounds such as astaxanthin, EPA, and DHA. Furthermore,the products cover both pharmaceutical drug delivery and health consumption scenarios. And the technology boasts deep barriers to entry and significant industrialization potential which setting a benchmark for the large-scale application of synthetic biology in the medical and health sectors.
项目名称/Project Name:
新机制全球创新小分子抗肿瘤免疫治疗药物开发
融资轮次/Funding Rounds:
Pre-A轮
项目亮点/Project Highlights
本公司开发的通过诱导肿瘤干细胞衰老而精准激活抗肿瘤免疫的小分子药物,较现有激活抗肿瘤免疫的抗体药物或细胞治疗策略相比,具有作用机制新颖、适应症更广泛、安全性更高、整体抗肿瘤记忆性、联用方案灵活等特点。一旦开发成功,必将为肿瘤免疫治疗增加一种高效可靠的方案,将彻底改变现阶段抗肿瘤免疫治疗格局,造福广大肿瘤患者。
Aobio is developing the small molecule oral drugs which precisely activates anti-tumor immunity by inducing the senescence of tumor stem cells. It has the characteristics of the novel mechanism, a wider range of indications, higher safety, overall antitumor memory after treatment,and flexible combination regimens compared with existing antibody drugs and cell-based therapy strategies for activating anti-tumor immunity;Once successfully developed, it will add an efficient and reliable option to tumor immunotherapy, significantly transform the current landscape of anti-tumor immunotherapy, and bring benefits to a large number of cancer patients.
项目名称/Project Name:
肿瘤诊疗一体化创新疗法
融资轮次/Funding Rounds:
Pre-B轮
项目亮点/Project Highlights
通过MRD高灵敏度动态监测精准预警复发风险,并基于此为高风险患者快速定制个性化mRNA肿瘤疫苗,形成“监测-评估-干预”闭环。该模式将前沿检测技术与创新疫苗平台深度融合,推动肿瘤治疗从传统“一次性治疗”转向“全周期慢病化管理”,致力于实现术后长期无病生存,为结直肠癌、肝癌等高复发瘤种提供覆盖预警、干预的个性化解决方案,是“诊疗一体化”战略在肿瘤慢病领域的标杆实践。
Based on the high-sensitivity dynamic monitoring of MRD, the recurrence risk can be precisely predicted, and personalized mRNA tumor vaccines can be quickly customized for high-risk patients, forming a closed loop of "monitoring - assessment - intervention". This model deeply integrates cutting-edge detection technology with an innovative vaccine platform, promoting the transformation of tumor treatment from traditional "one-time treatment" to "full-cycle chronic disease management", aiming to achieve long-term disease-free survival after surgery. It provides personalized solutions covering early warning and intervention for high-recurrence tumor types such as colorectal cancer and liver cancer, and is a benchmark practice of the "integrated diagnosis and treatment" strategy in the field of tumor chronic diseases.
项目名称/Project Name:
靶向GPC3的纳米抗体ADC药物 (NDC)
融资轮次/Funding Rounds:
天使轮+Pre-A轮
项目亮点/Project Highlights
公司依托高效的研发团队,利用纳米抗体制备技术和自建耦联平台的优势,开发了一款纳米抗体介导的ADC药物 ( 简称NDC), 并凭借其小而精, 可以穿透到肿瘤深处杀死肿瘤的独特机制,早期的有效性实验表明 在安全剂量范围内,既可以有效地抑制小肿瘤 ( 100--130 mm3) 95%, 又可以有效的抑制大肿瘤 ( 300-900 mm3) 达到90%, 急毒实验表明,小鼠接受6 倍的实验剂量注射, 没有表现出, 体重下降, 行为异常, 掉毛, 食欲改变等症状。 目前已经基本完成Pre-IND的准备工作, 进入IND的准备阶段。
1) 全球首个靶向GPC3的纳米抗体ADC药物
2) GPC3纳米抗体对大肿瘤的抑制率达到90%
3) GPC3纳米抗体具有超强的结合力KD= 1 pM
项目名称/Project Name:
FIC小分子细胞自噬与凋亡诱导剂治疗阿尔兹海默症、慢性癌痛和胃癌
融资轮次/Funding Rounds:
天使轮
项目亮点/Project Highlights
FIC系列小分子创新药在临床前实验中对阿尔兹海默症、慢性癌痛、胃癌和免疫疾病展现出优秀的药学活性,成药性优良。创新性靶向调控ROS使药物能选择性诱导病态细胞凋亡或重启神经细胞自噬。
团队成功的将黄酮类化合物衍生物生物利用度提高19倍,血药浓度提升682倍,成药性优良。依托于大连理工国家重点实验室,黄酮类化合物结构修饰与全化学合成技术国际领先。
其中CNS首创药能有效穿透血脑屏障,与OpenAI CEO山姆奥特曼投资的AD创新药RTR242部分机制和通路选择有相似性,是全球唯二在研发的mTOR抑制剂类自噬诱导剂阿尔兹海默症创新药。
项目名称/Project Name:
新一代AI驱动小分子设计平台及创新药开发
融资轮次/Funding Rounds:
天使轮
项目亮点/Project Highlights
我们致力于通过下一代人工智能重塑药物发现。由具有MNC背景的资深计算化学科学家领衔搭建的包括AI科学家,生物学家与药物化学家跨界组成的药物研发团队,开发国际领先的AI药物设计引擎,深度融合小分子生成式AI与物理计算,构建了分子生成与高通量自动合成优化相结合的端到端药物开发平台。目前已成功推进多个First-in-Class候选化合物进入临床候选阶段,显著缩短研发周期、降低开发成本、开启AI赋能新药研发的新纪元。
项目名称/Project Name:
人工智能大模型驱动的蛋白质设计
融资轮次/Funding Rounds:
A轮
项目亮点/Project Highlights
项目凭借自研蛋白质大模型,开创“AI设计+少量实验”新范式,解决研发周期长、多指标优化难等痛点。提供从设计、验证到商业化生产的全流程方案。已交付大量案例,覆盖医药及合成生物领域,获头部客户认可及多轮投资。
Project Highlights:
Project: AI-Driven Protein Design Leveraging a proprietary protein foundation model, this project pioneers an “AI Design + Minimal Validation” paradigm, solving bottlenecks in R&D cycles and multi-objective optimization. We provide end-to-end solutions from design to commercial production. With 30+ industrial cases delivered in biopharma and synthetic biology, our technology is validated by industry leaders and top-tier VCs.
项目名称/Project Name:
一种新型的用于治疗脑胶质瘤的双抗ADC分子
融资轮次/Funding Rounds:
Pre-A轮
项目亮点/Project Highlights
胶质母细胞瘤(GBM)是一种高侵袭性恶性脑肿瘤,患者 5 年生存率极低,这一困境主要源于两大核心治疗瓶颈:一是血脑屏障的物理阻隔,二是肿瘤本身存在高度异质性。本研究中研发的新型候选双特异性抗体药物偶联物DL332(靶向白介素 3 受体 α 链 × 人 CD98 重链),创新性利用 CD98 受体介导的转胞吞作用通路,有效提升了药物穿透血脑屏障的效率 —— 而血脑屏障穿透率低,正是传统单特异性抗体药物偶联物(ADC)治疗脑肿瘤的核心短板。
临床前研究数据充分验证了该药物的设计优势:小鼠体内实验显示,DL332 在脑组织中的药物浓度可达 16 nM,这一浓度是无 CD98 靶向能力的单特异性抗体药物偶联物 DL331的 10 倍。在原位胶质瘤模型中,药物脑部递送效率的提升,也与肿瘤生长抑制效果的显著增强呈正相关,这一结果凸显出该双靶点策略的应用潜力。综上所述,DL332可有效改善抗体药物偶联物在脑组织中的分布效率与抗肿瘤疗效,为胶质母细胞瘤的临床治疗开辟了极具前景的新方向。
Glioblastoma (GBM) is a highly aggressive malignant brain tumor with an extremely poor five-year survival rate, largely due to two major therapeutic obstacles: the blood-brain barrier, and the high heterogeneity of the tumor itself . In this context,
bispecific antibody-drug conjugates (BsADCs) represent an emerging therapeutic strategy designed to overcome these challenges by simultaneously targeting two distinct antigens or epitopes. Our new bsADC candidate, DL332 (IL3RA x hCD98hc), exploits the CD98 receptor-mediated transcytosis pathway to facilitate improved transport across the blood-brain barrier, a significant limitation for conventional monoclonal antibody-based ADCs . Preclinical studies substantiate this design advantage, demonstrating that DL332 achieves a brain concentration of 16 nM in mice—tenfold higher than the monospecific ADC DL331, which lacks CD98 targeting capability . This enhanced brain delivery correlated with superior suppression of tumor growth in an orthotopic glioma model, highlighting the potential of this dual-targeting approach . Consequently, DL332 exemplifies a rational BsADC design that improves the distribution and efficacy of ADC drugs within the brain, offering a promising therapeutic avenue for tackling glioblastoma.
项目名称/Project Name:
新一代工程化免疫启动生物制剂开发平台(In-Vivo/De Novo,诱导抗原特异性免疫响应)
融资轮次/Funding Rounds:
A轮
项目亮点/Project Highlights
新一代工程化免疫启动平台,In-Vivo/De Novo 诱导高活性、长记忆、安全平衡的抗原特异性免疫细胞,直接补充杀伤性免疫细胞储备池,作为突破实体冷肿瘤的免疫治疗基干(backbone);
非个体化、非 CGT 的通用型免疫疗法,具备高度 扩展性、工程化设计与规模化生产能力,降低临床与产业化门槛;
管线布局多个超级重磅适应症,首发产品已获临床安全性验证,核心产品陆续进入药效验证;由资深研发团队领衔,已完成头部机构领投的两轮累计近 3 亿元人民币融资,与多家潜在合作方开始接触并获得积极反馈。
A next-generation engineered immune priming platform that in vivo induces de novo high-potency, long-memory, and safety-balanced antigen-specific immune cells, directly replenishing the cytotoxic immune cell reservoir as a backbone for overcoming immune-cold solid tumors.
A non-individualized, non-CGT universal immunotherapy modality with high scalability, engineered design, and manufacturability, reducing clinical and industrialization barriers.
Pipeline spans multiple high-impact indications; the lead asset has demonstrated clinical safety, and core candidates are advancing through efficacy validation.
Led by a seasoned R&D team, the company has completed two financing rounds totaling ~RMB 300M led by top-tier investors, and is engaging multiple potential partners with positive feedback.
项目名称/Project Name:
新一代创新型装甲CAR巨噬细胞抗实体瘤的临床前研究
融资轮次/Funding Rounds:
种子轮+天使轮
项目亮点/Project Highlights
CAR巨噬细胞(CAR-M)能否用于临床肿瘤治疗,一是其能否实现在机体持久活化; 二是能否拮抗肿瘤微环境的免疫抑制M2极化胁迫。本项目全球首发全新“CAR”结构,实现真正意义符合巨噬细胞特性的CAR-M。原创设计特异适用于CAR-M的α1β1整合素介导FcγRⅠ胞内活化结构域激发在体持久抗肿瘤活性(ACT CAR-M技术),获得显著优于当前国际的CD3ζ CAR-M临床前抗肿瘤效果;进而,创新设计装甲型CAR-M技术体系确保CAR-M维持自身M1特性的同时并可逆转TME(TMER CAR-M),抗肿瘤效果进一步提升。自主知识产权所建立的新型第三代CAR-M技术体系亟待开展临床转化。
Whether CAR macrophages (CAR-M) can be used in clinical tumor therapy depends on two factors: whether they can achieve sustained activation in the body and whether they can counteract the immunosuppressive M2 polarization stress of the tumor microenvironment. This project has globally pioneered a novel "CAR" structure, enabling the development of CAR-M that truly aligns with macrophage characteristics. The original design specifically utilizes the α1β1 integrin-mediated FcγRⅠ intracellular activation domain to stimulate in vivo persistent antitumor activity (ACT CAR-M technology), achieving significantly superior preclinical antitumor efficacy compared to current international CD3ζ CAR-M standards. Furthermore, the innovative design of the armored CAR-M technology system ensures that CAR-M maintains its M1 characteristics while reversing TME (TMER CAR-M), further enhancing antitumor effects. The newly established third-generation CAR-M technology system with independent intellectual property urgently requires clinical translation.
项目名称/Project Name:
新机制全球创新小分子抗肿瘤免疫治疗药物开发
融资轮次/Funding Rounds:
Pre-A轮
项目亮点/Project Highlights
本公司开发的通过诱导肿瘤干细胞衰老而精准激活抗肿瘤免疫的小分子药物,较现有激活抗肿瘤免疫的抗体药物或细胞治疗策略相比,具有作用机制新颖、适应症更广泛、安全性更高、整体抗肿瘤记忆性、联用方案灵活等特点。一旦开发成功,必将为肿瘤免疫治疗增加一种高效可靠的方案,将彻底改变现阶段抗肿瘤免疫治疗格局,造福广大肿瘤患者。
Aobio is developing the small molecule oral drugs which precisely activates anti-tumor immunity by inducing the senescence of tumor stem cells. It has the characteristics of the novel mechanism, a wider range of indications, higher safety, overall antitumor memory after treatment,and flexible combination regimens compared with existing antibody drugs and cell-based therapy strategies for activating anti-tumor immunity;Once successfully developed, it will add an efficient and reliable option to tumor immunotherapy, significantly transform the current landscape of anti-tumor immunotherapy, and bring benefits to a large number of cancer patients.
项目名称/Project Name:
肿瘤诊疗一体化创新疗法
融资轮次/Funding Rounds:
Pre-B轮
项目亮点/Project Highlights
通过MRD高灵敏度动态监测精准预警复发风险,并基于此为高风险患者快速定制个性化mRNA肿瘤疫苗,形成“监测-评估-干预”闭环。该模式将前沿检测技术与创新疫苗平台深度融合,推动肿瘤治疗从传统“一次性治疗”转向“全周期慢病化管理”,致力于实现术后长期无病生存,为结直肠癌、肝癌等高复发瘤种提供覆盖预警、干预的个性化解决方案,是“诊疗一体化”战略在肿瘤慢病领域的标杆实践。
Based on the high-sensitivity dynamic monitoring of MRD, the recurrence risk can be precisely predicted, and personalized mRNA tumor vaccines can be quickly customized for high-risk patients, forming a closed loop of "monitoring - assessment - intervention". This model deeply integrates cutting-edge detection technology with an innovative vaccine platform, promoting the transformation of tumor treatment from traditional "one-time treatment" to "full-cycle chronic disease management", aiming to achieve long-term disease-free survival after surgery. It provides personalized solutions covering early warning and intervention for high-recurrence tumor types such as colorectal cancer and liver cancer, and is a benchmark practice of the "integrated diagnosis and treatment" strategy in the field of tumor chronic diseases.
项目名称/Project Name:
双机制的抗深部真菌感染PCC分子
项目亮点/Project Highlights
深部真菌感染每年造成375万人死亡,但是治疗药物有限,耐药问题突出。分子双重机制,抗菌谱广,已完成药效、安全性等验证,治疗窗口比目前临床一线药物提高了十倍以上。处于全面的临床前研究阶段。可开发多个适应症。
Deep fungal infections cause 3.75 million deaths annually, yet treatment options are limited and drug resistance presents a significant challenge. This molecule features a dual mechanism of action and wide anti-fungal spectrum. Its efficacy, safety and other properties have been verified by CROs, and the therapeutic window is more than ten times wider than that of current first-line clinical drugs. It is now in a comprehensive preclinical research phase and can be developed for multiple indications.
项目名称/Project Name:
模块化分合靶向蛋白降解技术
项目亮点/Project Highlights
提高降解剂生物利用度、体内作用时间、改善口服能力等成药参数;可通量筛选新颖E3连接酶配体,并将E3连接酶与目的蛋白进行最优配对;高效延展至除蛋白降解外其他临近诱导修饰领域,如磷酸化、去泛素化等。
Enhance the bioavailability of degraders, the duration of action in vivo, and improve oral administration and other drug parameters; High-throughput screening novel E3 ligase ligand, and E3 ligase optimal matching and target protein; Efficient extend to other proximity induced field except protein degradation, such as phosphorylation, to ubiquitin.
更多路演项目持续更新中……
已报名投资机构高层(部分)
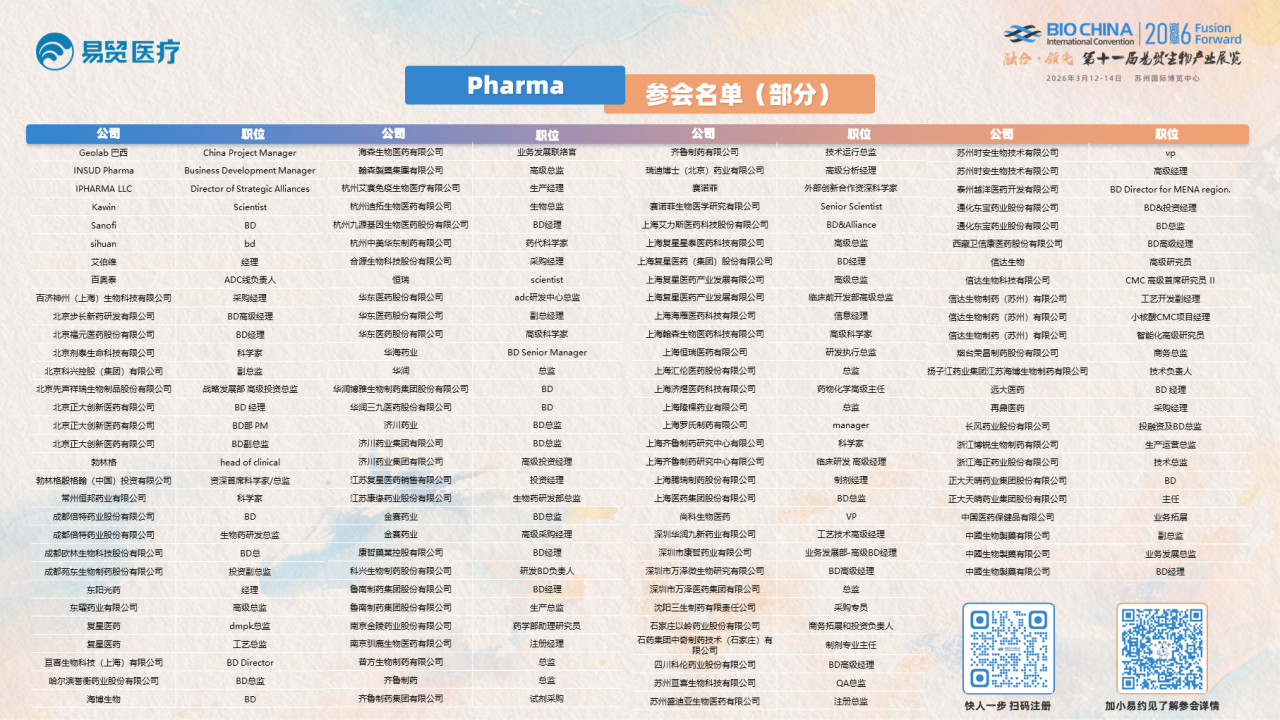
Pharma参会名单(部分)
投资人报名参与大会,即可高效对接优质项目,抢占产业投资风口,加速投资布局落地!
扫码注册门票
享多重福利
咨询路演详情
联系小易约见




